Cat: MF-2042
Cat: MF-2042
GM-CSF/CSF2, Mouse, HEK293 Cells,Tag Free: Product Information
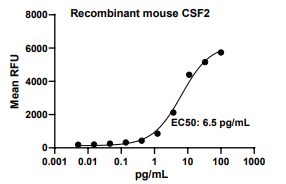
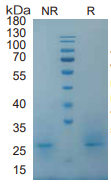
GM-CSF/CSF2, Mouse, HEK293 Cells,Tag Free:SDS-PAGE & Bioactivity
GM-CSF/CSF2, Mouse, HEK293 Cells,Tag Free:Synonyms
Colony-stimulating factor; CSF; CSF2; CSF-2; GMCSF; GM-CSF; Molgramostim; molgramostin
GM-CSF/CSF2, Mouse, HEK293 Cells,Tag Free:Background
CSF2 was initially characterized as a factor that can support the in vitro colony formation of granulocyte-macrophage progenitors. It is also a growth
factor for erythroid, megakaryocyte, and eosinophil progenitors. GM-CSF is produced by a number of different cell types (including T cells, B cells,
macrophages, mast cells, endothelial cells, fibroblasts, and adipocytes) in response to cytokine or inflammatory stimuli. On mature hematopoietic cells, GM-CSF is a survival factor for and activates the effector functions of granulocytes, monocytes/macrophages, and eosinophils (1, 2). GM-CSF
promotes a Th1 biased immune response, angiogenesis, allergic inflammation, and the development of autoimmunity (3-5). It shows clinical
effectiveness in ameliorating chemotherapy-induced neutropenia, and GM-CSF transfected tumor cells are utilized as cancer vaccines (6, 7). The
22 kDa glycosylated GM-CSF, similar to IL-3 and IL-5, is a cytokine with a core of four bundled alpha -helices (8-10). Mature mouse GM-CSF shares 49%-54% amino acid sequence identity with canine, feline, human, and porcine GM-CSF and 69% with rat GM-CSF. GM-CSF exerts its biological
effects through a heterodimeric receptor complex composed of GM-CSF R alpha /CD116 and the signal transducing common beta chain (CD131) which is also a component of the high-affinity receptors for IL-3 and IL-5 (11, 12). In addition, GM-CSF binds a naturally occurring soluble form of GM-CSF R alpha (13).
1. Martinez-Moczygemba, et al. (2003) J. Allergy Clin. Immunol. 112:653.
2. Barreda, D.R. et al. (2004) Dev. Comp. Immunol. 28:509.
3. Eksioglu, E.A. et al. (2007) Exp. Hematol. 35:1163.
4. Cao, Y. (2007) J. Clin. Invest. 117:2362.
5. Fleetwood, A.J. et al. (2005) Crit. Rev. Immunol. 25:405.
6. Heuser, M. et al. (2007) Semin. Hematol. 44:148.
7. Hege, K.M. et al. (2006) Int. Rev. Immunol. 25:321.
8. Kaushansky, K. et al. (1992) Biochemistry 31:1881.
9. Diederichs, K. et al. (1991) Science 254:1779.
10. Gough, N.M. et al. (1984) Nature 309:763.
11. Onetto-Pothier, N. et al. (1990) Blood 75:59.
12. Hayashida, K. et al. (1990) Proc. Natl. Acad. Sci. 87:9655.
13. Pelley, J.L. et al. (2007) Exp. Hematol. 35:1483.